Kemwell’s Approach To Scale-Up Of Fed-Batch Cell Culture Processes
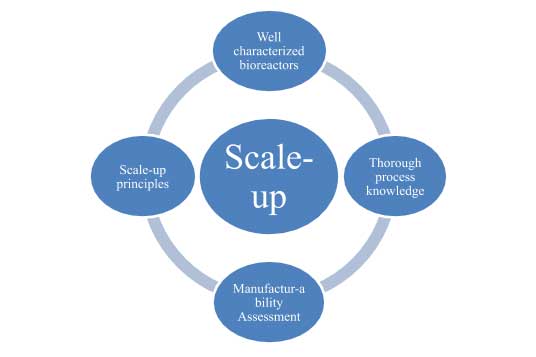
Kemwell, a leading contract manufacturing organization, has been working on projects from different customers, each with its own set of challenges. To tackle these distinct challenges it is important to have a holistic approach to scale-up. The recipe for successful scale-up of a mammalian cell culture process is provided in the figure below.
Well characterized bioreactors: With the variety of processes we encounter, it is imperative that we fully understand the capability of our bioreactors from bench-scale to large scale. All bioreactors at Kemwell are therefore characterized thoroughly for:
1. Oxygen transfer – Simply put, the bioreactor should be capable of supporting the cell density requirements of a process. Sufficient oxygen supply is important for reproducing the same cell growth profile at different scales. The oxygen mass transfer coefficient kLaO2 has been determined with different sparger and impeller configurations, which will be useful in assessing the oxygenation requirements based on the process requirements. Based on the data generated, the bioreactors at Kemwell are capable of supporting cell densities up to 50 million cells/mL and beyond.
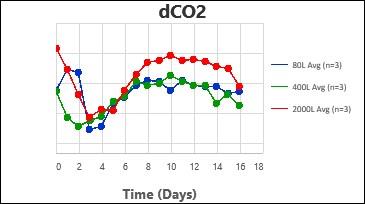
2. Carbon dioxide removal – Higher levels of dissolved CO2 were shown to be detrimental to cell growth and productivity by different groups. The dCO2 removal rate (kLaCO2) was evaluated at different agitation speeds and air sparge rates for each scale, with different type of spargers.
3. Bulk mixing – Inefficient mixing can lead to pH and nutrient gradients in large scale bioreactors resulting in poor cell growth and productivity. Mixing times were determined at different volumes and different agitation speeds and found to be comparable to those published in literature. As an example, in the 2000L bioreactor, the mixing time was determined to be 54 sec with an agitation speed of 70 rpm.
Thorough process knowledge: It is important to build a thorough knowledge about the process at small-scale, prior to scaling up. Our MSAT team is involved in discussions pertaining to the robustness of the process, thereby assessing the sensitivity of the process to parameters listed below. Developing an understanding of these parameters at an early stage, coupled with testing for process robustness helps significantly during scale-up.
1. Hydrodynamic parameters such as aeration and agitation
2. Environmental factors such as temperature and pH
3. Dissolved carbon dioxide
4. Metabolic parameters such as glucose uptake and lactate production
Manufacturability assessment: With the different types of processes handled by a CMO, manufacturability assessment is a vital part of scale-up. This assessment performed by the MSAT and operations team has two outcomes.
1. Identify additional equipment that may be required to fit the process being developed/transferred at small-scale. The assessment must be completed in the initial stages of technology transfer to enable enough time to procure and qualify the equipment.
2. Modifications to the existing set up to enable the process execution. For example, recently, a cell culture process transferred by one of the clients had more than the usual number of nutrient feed additions. The number of feeds outnumbered the number of ports available for feeding. Therefore, additional aseptic connections had to be made in a manner that the sterility of these feeds is not compromised.
Scale-up principles: Although a lot of information is available in literature describing the scale-up considerations, one has to still integrate different approaches and tailor them to the specific situation. Listed below are some of the considerations, which we have successfully used at Kemwell.
1. The bioreactor geometry is similar across the pilot scale and large scale bioreactors. Parameters such as aspect ratio and impeller to tank diameter ratio are kept constant across scales.
2. Ensuring similar power per unit volume across scales by adjusting the agitation and aeration rates.
3. Ensuring similar oxygen transfer rates across scales by leveraging the models built during bioreactor characterization studies.
4. Using a hybrid approach, where the theoretical kLa required for the process is estimated using the oxygen uptake rate for the cell line. Then, using the kLa model equations for the respective bioreactors, the P/V and gas flow rates required to meet the kLa are predicted iteratively.
5. Estimation of shear by using empirical equations.
6. The first scale-up always is at a pilot-scale in PD. This approach reduces the risks of failure by identifying potential problems early and mitigate them for larger scale.
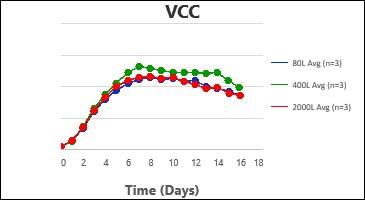
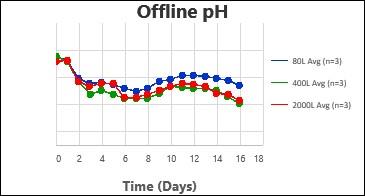
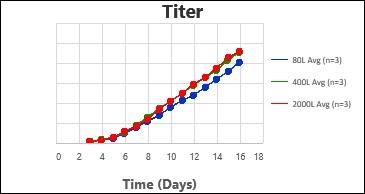
By following this comprehensive methodology, several processes have been successfully scaled up at Kemwell. An example is provided below, where a process was scaled up to 80L in pilot, followed by 400L for Phase I manufacturing and 2000L for Phase III manufacturing. The key attributes were all comparable across the scales, thus validating the scale-up methodology followed at Kemwell.
Kemwell has successfully manufactured material for pre-clinical, clinical Phase I and Phase III studies at 400L and 2000L scales for India as well as US markets.
Categories
New at Kemwell
A collection of stories about our people, our capabilities, our research,
and the ever-changing face of our firm.
Business Wire release Milestone expands India’s role in global biologics manufacturing;…
Introduction: Recent advances in biologics development are primarily focussed on development…
Introduction: Size variants determination is a critical quality attribute for a…





